ILC2s as Drivers of Asthma Susceptibility
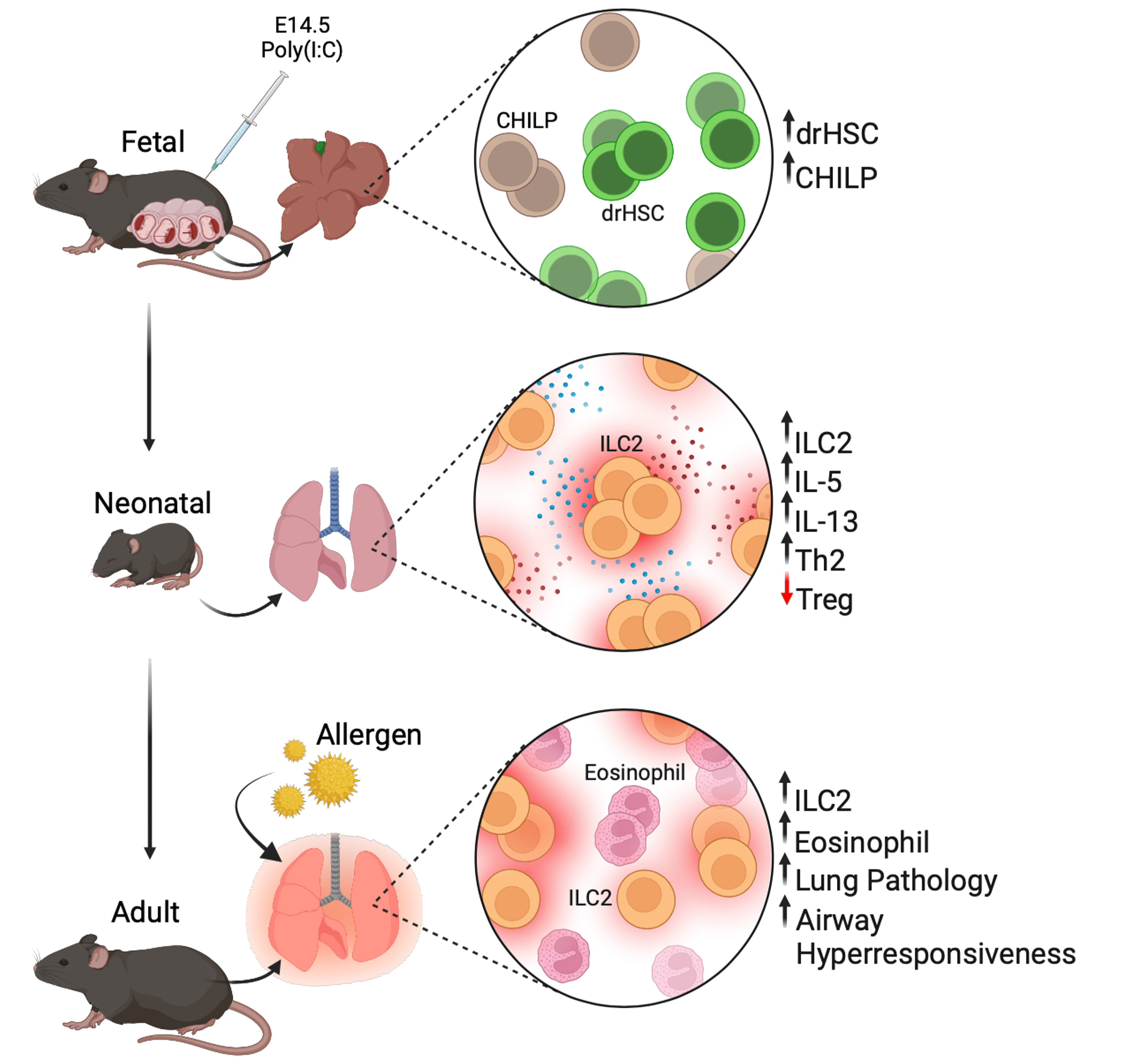
ILC2s are critical mediators of allergic asthma (AA), particularly in pediatric asthma. Viral infection during early life is strongly associated with increased risk for pediatric asthma and asthma susceptibility across the lifespan. Importantly, ILC2s are key players in viral-induced asthma pathogenesis, but the mechanisms by which ILC2s may promote lifelong lung dysfunction are poorly understood. ILC2s are among the earliest immune cells to seed and be functional in the developing lung, become tissue-resident, and show tremendous plasticity in their response to tissue and immune stimuli. Our published data reveal that prenatal inflammation induced in a mouse model with the TLR3 agonist poly(I:C) drives sustained changes to the lung ILC2 compartment and permanent developmental consequences for lung immunity and lung function. Importantly, our data indicate that prenatal inflammation programs hyperactive ILC2 development from a fetal hematopoietic progenitor, indicating a novel developmental mechanism
In ongoing work, we aim to capitalize on our findings that early life inflammation can drive the hyper-activation of fetal-derived immune cells to directly test:
- the mechanism by which prenatal inflammation programs the phenotype and persistence of functionally altered ILC2s in the postnatal lung
- the requirement for reprogrammed ILC2s in shaping lung immunity and lung dysfunction
- whether a comparable phenomenon exists during human development that can be leveraged as a biomarker for understanding impaired immune development and asthma risk in response to early-life infection